What You Need to Know-Week of November 1st
The most important weekly updates for you to keep your community healthy

In this Update:
1. Urgent Updates: COVID-19 Metrics
2. All Things Vaccines: Moderna and J&J Booster Shots
3. All Things Vaccines: Pfizer Vaccine recommended for children aged 5 – 11
1. Urgent Updates: COVID-19 Metrics
Updated as of: 11-01-2021
Weekly case, death, and hospitalization counts
| In the past week, there has been an average of: | |
| Cases per day | 3,001 |
| Deaths per day | 50 |
| Compared to two weeks ago: | |
| Cases per day | Increased by 81% ↑ |
| Deaths per day | Increased by 126% ↑ |
| Hospitalizations per day | Increased by 4% ↑ |
View all data related to covid19 in arizona state
Hospital Capacity Metrics:
| Percent of Arizona hospital beds currently in use | 94% |
|---|---|
| Percent of Arizona hospital beds currently in use by COVID-19 patients | 27% |
Vaccine Information:
| Number of Arizonans | Percentage of Arizonans | Percentage in United States | |
|---|---|---|---|
| Arizonans who are fully vaccinated | 3,788,100 | 53% | 58% |
| Arizonans who have received at least one dose (of a Pfizer or Moderna vaccine) |
4,259,681 |
61% |
67% |
8,276,606 total COVID-19 vaccine doses have been administered in Arizona
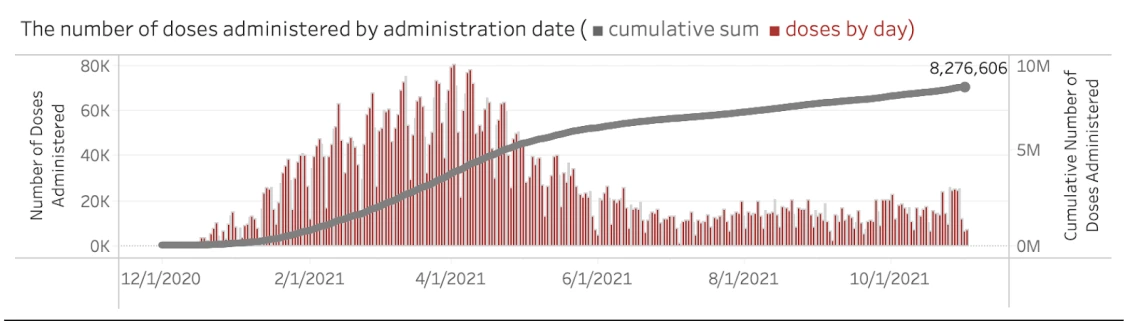
See breakdown of vaccination by Arizona counties
See updated vaccine information and data in Arizona
2. All Things Vaccines: Pfizer Vaccine approved for children aged 5 – 11
The FDA and CDC have endorsed and recommended a smaller dose of the Pfizer vaccine for children between the ages of 5 and 11 as of October 26th!
Children 5 – 11 will be able to receive a COVID-19 vaccine at their pediatrician’s office or pharmacies. Vaccine appointments for the weekend are already becoming available to book.
The children’s dose, one-third of an adult dose, has successfully undergone rigorous clinical trials and has been proven to be both safe and effective. The side effects reported during the trials for 5 – 11 year olds were mainly mild and comparable to those experienced by children and young adults 16 – 25.
Learn more about COVID-19 Vaccines for Children and Teens
3. All Things Vaccines: Moderna and J&J Booster Shots
Moderna
Moderna recently requested authorization for high risk groups (e.g., adults over 65 years old) to receive the booster, similar to the recent recommendations for Pfizer boosters. On October 14, the FDA reached a unanimous decision to recommend emergency authorization of a booster shot for those who meet the outlined criteria below.
- Booster eligibility criteria
- Individuals who have completed the two-dose Moderna vaccine series at least six months prior AND either are:Over the age of 65 OR areBetween 18 – 64 years old and are at high risk of developing severe COVID-19, or are at high risk for being exposed to COVID-19 in their job setting.
J&J
The Food and Drug Administration (FDA) advisory committee also unanimously voted to authorize second shots for all those who had received the one-shot J&J vaccine.
- Booster eligibility criteria
- Those who had previously received the one-shot vaccination at least two months prior AND are 18 or older
CDC recommendations now also allow for mixing and matching of booster shots across the different available vaccines! See FDA article for more information.
The next update will cover information on a study showing the importance of masks within Arizona schools. If you would like to learn more about this and other topics related to COVID-19 in Arizona, please complete next week’s AZCOVIDTXT survey that you will receive via text in about a week.
